Paraganglioma:
Now we come to a finding that I think was underappreciated. Mr. Floyd had a small pelvic tumor that, on evaluation, was diagnosed as a benign paraganglioma. Here’s the key. While the tumor was benign, there’s a reasonable chance that it was a “functional” tumor. Tumors, both benign and malignant, often retain some similarity to the tissues in which they arose. A tumor of the bronchial epithelium may still look like bronchial epithelium — but it is disorganized and grows where it shouldn’t.
Tumors of organs that secrete hormones may still secrete those hormones or variations of them. When they do, the patient may have an excess of the hormone which may become symptomatic. Because the tumor contains damaged cells, the product may be somewhat different than the naturally produced hormone, and may have different effects. When these effects are symptomatic, it’s called a “paraneoplastic syndrome.”
Paragangliomas are tumors of cells that, for the most part, produce “catecholamines” — stimulatory hormones such as adrenalin (though some, those of the head and neck can are so-called “parasympathetic” paragangliomas and secrete a different hormone). When the tumor is found in the adrenal gland, it is called a “pheochromocytoma.” When it is found in a different part of the body, it is called a “paraganglioma.” Almost all paragangliomas outside of the head and neck actively secrete hormone. There is an old mnemonic that says that about 10% of paragangliomas secrete enough hormone to be symptomatic, more resent studies suggest that it’s more than that. With respect to pelvic paragangliomas (which is what Mr. Floyd had), it’s likely much more. In one small study of 7 such tumors, 4 were associated with elevated catecholamine levels, 3 noted before surgery and one during surgery (1). I saw no statements that the functional status of Mr. Floyd’s tumor had been evaluated. When functional, these tumors can constantly produce large amounts of hormone, small amounts of hormone, or be “paroxysmal,” meaning that they secrete hormone in spurts leading to sudden attacks of symptoms.
The symptoms of a functional paraganglioma are well known. Here’s a description from one review article(2):
Paragangliomas that hypersecrete catecholamines may cause signs and symptoms identical to those in patients with hyperfunctioning adrenal pheochromocytoma. Episodic symptoms may occur in spells, or paroxysms, that can be extremely variable in presentation but typically include forceful heartbeat, pallor, tremor, headache, and diaphoresis. The spell may start with a sensation of a “rush” in the chest and a sense of shortness of breath, followed by a “pounding” heartbeat in the chest that typically progresses to a throbbing headache. Peripheral vasoconstriction with a spell results in cool/cold hands and feet and facial pallor. Increased sense of body heat and sweating are common symptoms that occur toward the end of the spell. Spells may be either spontaneous or precipitated by postural change, anxiety, medications (e.g., metoclopramide, anesthetic agents), exercise, or maneuvers that increase intra-abdominal pressure (e.g., change in position, lifting, defecation, exercise, colonoscopy, pregnancy, trauma)
Here’s another description(3):
Patients with pheochromocytoma present with the signs and symptoms of catecholamine excess. The classic triad of symptoms consists of episodic headache (72%), sweating (69%), and palpitations (51%). Other signs and symptoms that can occur include pallor, anxiety, flushing, visual blurring, polyuria, polydipsia, weight loss, papilledema, orthostatic hypotension, elevated erythrocyte sedimentation rate, hyperglycemia, psychiatric disorders, dilated cardiomyopathy, stroke, or secondary erythrocytosis…
In addition to excessive amounts of unregulated catecholamines, pheochromocytomas have also been found to secrete other hormones, such as neuropeptide Y, parathyroid hormone related protein (PTH-rp), calcitonin, adrenocorticotropin hormone (ACTH), neuron specific enolase, interleukin-6 (IL-6), vasoactive intestinal peptide (VIP) and chromogranin A.
People with functional paragangliomas are more prone to panic attacks and stress cardiomyopathy, which I’ll discuss in a minute.
Self medication for the hyperadrenergic state:
So, what would you expect from someone who is living a life with a tumor that is, in essence, secreting home-grown methamphetamine? You’d expect chronic high blood pressure, excitability, perhaps a bit of impulse control issues. And, maybe, someone might self-medicate with opiates. Interestingly, this was brought out by proponents of the prosecution who were trying to squash evidence of Mr. Floyd’s drug use. One news report noted(4):
Floyd family attorneys Ben Crump and Antonio Romanucci released a statement after Ross’s testimony blasting the defense’s efforts bring Floyd’s drug use to the forefront of the case.
‘As the defense attempts to construct the narrative that George Floyd’s cause of death was the Fentanyl in his system, we want to remind the world who witnessed his death on video that George was walking, talking, laughing, and breathing just fine before Derek Chauvin held his knee to George’s neck, blocking his ability to breathe and extinguishing his life for all to see,’ the statement read.
‘Tens of thousands of Americans struggle with self-medication and opioid abuse and are treated with dignity, respect and support, not brutality. We fully expected the defense to put George’s character and struggles with addiction on trial because that is the go-to tactic when the facts are not on your side.
Now it may be that the functional status of Mr. Floyd’s tumor was assessed, but such results were not shared during discovery prior or during the trial as far as I know. Since I believe that this paraganglioma might have been very important, I concur with the family’s description of the Mr Floyd’s use of drugs as “self-medication.” It makes sense to me that he was self-medicating for the effects of his tumor. I’ll write a bit more about this in the next installment.
Stress cardiomyopathy:
But here’s what is important with respect to this case. This excess catecholamine can result in sudden cardiac failure and death, particularly under stress. In one study of 140 consecutive patients with paraganglioma/pheochromocytoma, 11% suffered acute catecholamine cardiomyopathy (ACC). The authors write(5):
ACC presented as abrupt cardiorespiratory failure in all 15 subjects. Twelve patients suffered acute pulmonary oedema, associated with chest pain in five cases. In 10 of these 12 patients, ACC progressed to cardiogenic shock requiring inotropic treatment and invasive ventilation, associated with intra-aortic balloon counter pulsation, extracorporeal membrane oxygenation or continuous veno-venous haemofiltration in three patients. The three patients without pulmonary oedema presented at the emergency room with sustained oppressive chest pain with stable haemodynamics (two) or heart failure (one). Twelve patients had high BP (170 (163–220)/110 (108–110) mm Hg) and 10 had a high heart rate (140 (129–149) beats/min (bpm)). Three patients were normotensive (BP 120 (118–127)/ 69 (69–73) mm Hg), including two with tachycardia (heart rate 132 and 140 bpm). Seven patients presented with sinus tachycardia, one with paroxysmal supraventricular tachycardia, and one with atrial flutter. Severe ventricular arrhythmias occurred in three patients, two with non-sustained episodes of ventricular tachycardia and one with ventricular tachycardia that evolved to ventricular flutter; this last patient and another patient who developed asystole had been successfully resuscitated after cardiac arrest.
So, stress can trigger this. And, in fact, this is sometimes called “stress cardiomyopathy.”
There is some wordsmithing to be done here. Classic “stress cardiomyopathy” is also called Takotsubo cardiomyopathy, and is not associated with paragangliomas. Moreover, the technical definition of Takotsubo cardiomyopathy requires the absence of coronary artery disease in some definitions, though this seems to be increasingly disputed. Thus, some people include paraganglioma-associated heart failure in the general term “stress cardiomyopathy” and some exclude it and use it only for Takotsubo cardiomyopathy. For instance, Y-Hassan and Falhammar write(5) “Takotsubo syndrome (TS), also known as neurogenic stunned myocardium or broken heart syndrome, is a recognized acute cardiac syndrome. In about 70% of cases, the syndrome is preceded by an emotional or a physical stressor. Among the innumerable physical trigger factors that may induce TS are pheochromocytomas and paragangliomas (PPGLs).”
Similary Gagon et al note (6)” Acute TLC may be found in up to 3% of patients with secreting PPGL.” Where “TCL stands for “Takotsubo-like catecholamine cardiomyopathy” and “PPGL” stands for “Pheochromocytoma and paraganglioma).
Further, in patients with coronary artery disease, such as Mr. Floyd, physiologic stress associated with traditional ischemia, such as from exertion with severe coronary artery disease, can trigger stress cardiomyopathy in areas not in the watershed of the affected artery (7).
So, this is “Takotsubo-like cardiomyopathy” rather than “Takotsubo cardiomyopathy.”
Whatever you call it, the common pathway is the combination of stress and catecholamines. As one article notes(8): “Whatever the trigger, the common denominator in TTC is catecholaminergic stress. Classically suggested after emotional trauma, TTC may also be induced by surgical stress or endogenous or iatrogenic 2-mimetic intoxication.”
For the purpose of this discussion, I’m going to be a lumper, because both can be induced by stress. For for the general use of the term, it turns out that people with cancer are more likely to suffer stress-related cardiomyopathy, and people with stress-related cardiomyopathy are more likely to have tumors (though not necessarily paraganglioma)(9).
Can other things than physical and emotional stress do it? Why yes, yes they can. As you might expect, if a person is producing too much catecholamine, then taking stimulant drugs might make it much much worse. And in fact, that’s the case. If you give a person with a paraganglioma a drug like that you can induce stress-cardiomyopathy-like heart failure. That includes amphetamines, serotonin re-uptake inhibitors, and others. Including methamphetamine(10). Mr. Floyd was both self-medicating and exacerbating his chance of stress cardiomyopathy by the combination of drugs in his system.
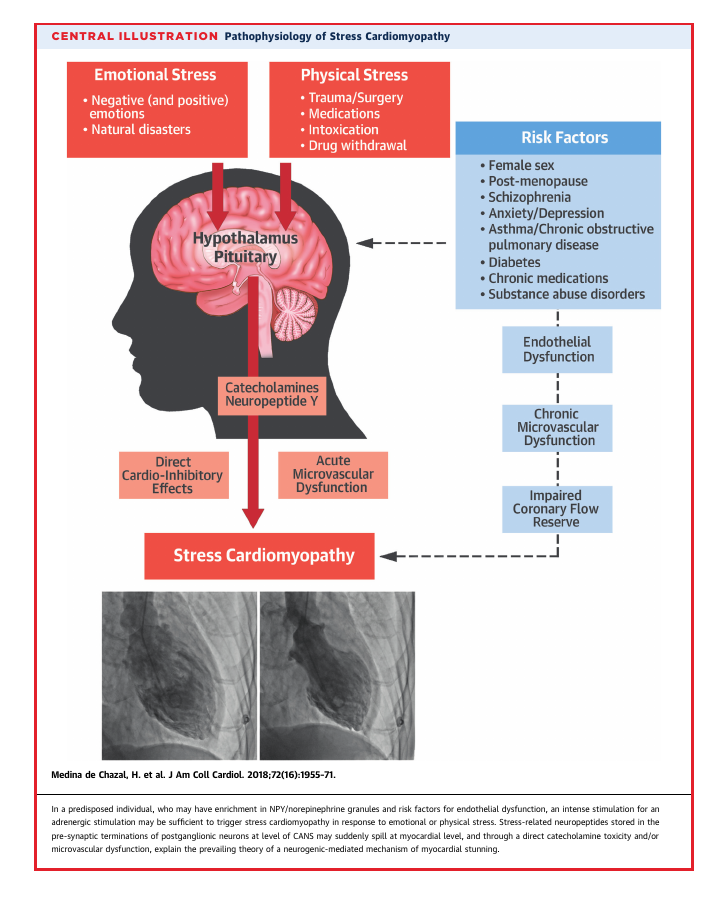
Here’s a nice graphic from a JACC review (11):
This is mediated through the hormonal and autonomic nervous systems. Here’s another great graphic from the JACC folk (12):
Now, traditionally, Takotsubo cardiomyopathy is considered reversible and rarely, if ever fatal. But more recently that’s changed. One study found that people who suffered stress cardiomyopathy had a higher 1-year mortality than matched patients who had suffered myocardial infarction (Hazard ratio 1.58). The authors note ( where “Killip class” is a 0-4 classification to predict outcome in a heart attack, and LV is left ventricle)(13):
Predictors of mortality using univariable Cox regression analysis were age >70 years, male sex, apical ballooning, a physical stressor, Killip class 3/4 on admission, initial LV ejection fraction <40%, and diabetes mellitus (Table 3). On multivariable analysis, male sex (HR 1.97, 95% CI 1.03–3.78; P = 0.04), Killip class 3/4 (HR 6.03, 95% CI 3.26–11.17; P < 0.01), and diabetes mellitus (HR 2.11, 95% CI 1.23–3.65; P < 0.01) remained significant predictors of long-term mortality.
These three dichotomous traits were used to calculate a risk score by allocating one point for the presence of each factor. Mortality rates in patients exhibiting risk scores from 0 to 3 points were 13.7%, 37.3%, 64.7%, and 100%, respectively.
It should be noted that the cause of death in these cases was not noted.
In one large study, the mortality of people with stress cardiomyopathy was essentially the same as that with more conventional ischemic heart attacks acutely. The authors of that study found (where STEMI stands for “ST elevation myocardial infarction”, and NSTEMI is “non-ST elevatopm myocardial infarction”)(14):
“The proportion of the patients diagnosed with takotsubo increased from 0.16% in 2005 to 2.2% in 2012 (P b 0.05); 14% of these patients also had significant coronary artery disease. Cardiogenic shock developed more frequently in patients with takotsubo than NSTEMI (adjusted OR 3.08, 95% CI 1.80–5.28, P b 0.001). Thirty-day mortality was 4.1% and was comparable to STEMI and NSTEMI. The long-term risk of dying from takotsubo (median follow-up 25 months) was also comparable to NSTEMI (adjusted HR 1.01, 95% CI 0.70– 1.46, P = 0.955) STEMI (adjusted HR 0.83, 95% CI 0.57–1.20, P = 0.328).”
The authors conclude:
“Patients with takotsubo syndrome have short- and long-term mortality rates similar to those of patients with acute myocardial infarction and often have concomitant advanced coronary artery disease.”
Similarly, Pellicia et al write(15): The prognosis was initially thought to be benign, but subsequent studies have demonstrated that both short-term mortality and long-term mortality are higher than previously recognized. Indeed, mortality reported during the acute phase in hospitalized patients is ≈4% to 5%, a figure comparable to that of ST-segment–elevation myocardial infarction in the era of primary percutaneous coronary interventions.
Patients with coronary artery disease and stress-cardiomyopathy do much worse than those who do not have vulnerable hearts. One study notes (TSC = Takotsubo cardiomyopathy, CAD = Coronary artery disase) (16):
Being a patient with TSC was associated with a hazard ratio of 2.1 for death compared with the control subjects without CAD (95% confidence interval: 1.4 to 3.2). This was similar to the excess mortality risk seen among the CAD control subjects compared with control subjects without CAD (hazard ratio: 2.5; 95% confidence interval: 1.8 to 3.3). These associations remained significant after adjusting for CAD risk factors and risk markers for TSC.
There are a few racial differences that studies have discovered with respect to stress cardiomyopathy. Compared to Caucausians, African-Americans have about the same mortality. However, there is some evidence that African-Americans, or at least African-American women, are more likely to have shortness of breath as a primary symptom.
As one report notes (17):
Although chest pain is described as a cardinal feature in takotsubo cardiomyopathy, existing data suggest that African American patients may lack this typical symptom. The first African American female reported with takotsubo syndrome presented with heart failure and hypotension in the absence of chest pain. Subsequently, Patel et al. reported 5 African American women with takotsubo syndrome. Three patients presented with dyspnea, and 2 presented with nausea: none of the patients experienced chest pain. Our case adds to this evidence by describing an African American woman with takotsubo syndrome whose presenting symptom was severe dyspnea without chest pain. Unlike the majority of reported cases, electrocardiographic and biomarker abnormalities were not present in our patient at admission. As with our patient, the diagnosis of takotsubo cardiomyopathy may initially be overlooked in African Americans because of the atypical presentation. As takotsubo syndrome becomes increasingly recognized in the United States, clinicians are encouraged to consider the diagnosis in African American women who present with severe dyspnea in the setting of extreme emotional or physiological stress. Further research on the pathophysiology of takotsubo cardiomyopathy is needed to explain why such differences in presenting symptoms may exist.
The Patel article is reference 18.
I want to emphasize here that while paragangliomas increase the risk of stress cardiomyopathy, they are not necessary. It has been hypothesized that catecholamine-induced cardiomyopathy may be present in the context of restraint even in people without this tumor(19). Chronic hypertension, as present in Mr. Floyd, increases the risk of stress cardiomyopathy, in part because it’s a symptom of pheochromocytoma/paraganglioma. Hypertension is also associated with a worse outcome, primarily due to stroke (20). So, if Mr. Floyd’s paraganglioma was not functional, it doesn’t change the general pathway of his death. If his paraganglioma *was* functional, it’s important icing in the cake.
I’ll go into this in more detail when I put it all together in this case, but if you recognize Mr. Floyd’s reaction to being put into the police car as the beginning of a panic-type attack and an incipient stress reaction, then the remainder of the event is pretty much a textbook case of the clinical relationship between stress, vulnerable heart, intoxication in a person with high endogenous catecholamines.
In the next installment, I’ll discuss the drugs Mr. Floyd had taken.
- Ali-el-Dein B, el-Sobky E, el-Baz M, Shaaban AA. Abdominal and pelvic extra-adrenal paraganglioma: a review of literature and a report on 7 cases. In Vivo. 2002 Jul-Aug;16(4):249-54. PMID: 12224134.
- Young, W. F. (2006). Paragangliomas: Clinical Overview. Annals of the New York Academy of Sciences, 1073(1), 21–29. doi:10.1196/annals.1353.002)
- Yeo, Heather; Roman, Sanziana Pheochromocytoma and functional paraganglioma, Current Opinion in Oncology: January 2005 – Volume 17 – Issue 1 – p 13-18 doi: 10.1097/01.cco.0000147900.12325.d9
- https://www.dailymail.co.uk/news/article-9428573/Derek-Chauvin-trial-Jury-hears-new-audio-cop-speaking-George-Floyds-death.html
- Y-Hassan S, Falhammar H. Pheochromocytoma- and paraganglioma-triggered Takotsubo syndrome. Endocrine (2019) 65:483-493
- Gagnon N, Mansour S, Bitton Y, Bourdeau I. Takotsubo-like cardiomyopathy in a large cohort of patients with pheochomrocytoma and paraganglioma. Endocr Pract 2017:23:1178-1192.
- Rendón, Iliana S. Hurtado, et al. “Acute myocardial infarction and stress cardiomyopathy are not mutually exclusive.” The American Journal of Medicine 131.2 (2018): 202-205.
- Coupez E, Eschalier R, Pereira B, et al. A single pathophysiological pathway in Takotsubo cardiomyopathy: Catecholaminergic stress. Archives of Cardiovascular Disease (2014) 107, 245—252 http://dx.doi.org/10.1016/j.acvd.2014.04.001
- Sattler, K.; El-Battrawy, I.; Lang, S.; Zhou, X.; Schramm, K.; Tülümen, E.; Kronbach, F.; Röger, S.; Behnes, M.; Kuschyk, J.; Borggrefe, M.; Akin, I. (2017). Prevalence of cancer in Takotsubo cardiomyopathy: Short and long-term outcome. International Journal of Cardiology, (), S0167527317311233–. doi:10.1016/j.ijcard.2017.02.093
- Abraham J, Mudd JO, Kapur N, et al. Stress Cardiomyopathy After Intravenous Administration of Catecholamines and Beta-Receptor Agonists J Am Coll Cardiol 2009;53: 1320–5
- Medina de Chazal, Horacio, et al. “Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review.” Journal of the American College of Cardiology 72.16 (2018): 1955-1971.
- Lyon, Alexander R., et al. “Pathophysiology of Takotsubo syndrome: JACC state-of-the-art review.” Journal of the American College of Cardiology 77.7 (2021): 902-921.
- Stiermaier T, Moeller C, Oehler K, et al. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences European Journal of Heart Failure (2016) 18, 650–656 doi:10.1002/ejhf.494
- Redors B, Vedad R, Angeras O, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial
infarction — A report from the SWEDEHEART registry International Journal of Cardiology 185 (2015) 282–289 - Pellicia F, Kaski JC, Crea F, Gamici PG. Pathophysiology of Takotsubo Syndrome Circulation. 2017;135:2426–2441. DOI:10.1161/CIRCULATIONAHA.116.027121
- Tornvall P, Collse O, Ehrenborg E, Jarnbert-Petterson H. A Case-Control Study of Risk Markers and Mortality in Takotsubo Stress Cardiomyopathy J Am Coll Cardiol 2016;67:1931–6
- Pezzo, Stephanie P., Gregory Hartlage, and Charles M. Edwards. “Takotsubo cardiomyopathy presenting with dyspnea.” Journal of Hospital Medicine 4.3 (2009): 200-202.
- Patel HM, Kantharia BK, Morris DL, Yazdanfar S. Takotsubo syndrome in African American women with atypical presentation: a single-center experience. Clin Cardiol 2007;30:14-8.
- Krexi L, Georgiou R, Krexi D, Sheppard MN. Sudden cardiac death with stress and restraint: The association with sudden adult death syndrome, cardiomyopathy and coronary artery disease. Medicine, Science and the Law. 2016;56(2):85-90. doi:10.1177/0025802414568483
- Liang, Jing, et al. “Conventional cardiovascular risk factors associated with Takotsubo cardiomyopathy: A comprehensive review.” Clinical Cardiology 44.8 (2021): 1033-1040.